BRIST-IVF (Bristol IVF Study): Improving IVF outcomes
You are invited to take part in this research study which is aimed at assessing factors affecting the outcome of In-Vitro Fertilisation (IVF)/Intra-Cytoplasmic Sperm Injection (ICSI) treatment.
This study is being conducted in order to improve overall success rates of IVF/ICSI cycles and to gain greater understanding of any longer-term impacts on health.
We recognise that many factors can affect IVF/ICSI success rates but the impact of maternal and paternal health has not been well studied. In addition, relatively little is known about the longer-term outcomes of IVF/ICSI on parental and child health. The purpose of this study is to identify whether both maternal and paternal general health and lifestyle behaviours influence the outcome of IVF/ICSI and to assess the short and long-term impact of IVF treatment on parental and child health.
Who can participate?
Anyone (aged 18 or over) undergoing IVF/ICSI treatment at the BCRM. We want to collect information on all individuals involved in treatment including male and female partners.
Do I/we have to take part?
This study is voluntary and it is entirely up to you if you want to take part. If you choose to take part, you will be asked to keep this information sheet and to sign a consent form. You are free to withdraw from the study at any time without giving a reason and with no impact on the care you receive. Before you decide whether to take part, please take your time and carefully read the following information about the study. It is important that you understand what the study involves and what will be required. Please feel free to discuss it with your friends, family and health care team before deciding whether to take part. If you have any query or require further information, please do not hesitate to contact us.
Thank you very much for taking the time to read this.
| Contact information For more information, any doubts or questions, please feel free to contact the research team
Email: ivf-study@bristol.ac.uk Telephone: 07929 044 873 |
This study is funded and supported by the National Institute for Health Research Biomedical Research Centre (NIHR BRC) at University Hospitals Bristol NHS Foundation Trust and the University of Bristol. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. PIS_1 /Version 9.0/1 December 2021 IRAS ID: 236773
What do I/we have to do?
During the study, you will follow your clinical treatment protocol. The study does not require you to have any changes to your treatment plan. We will collect data from you at one of your treatment appointments, online or via telephone so you will not need to make additional visits to the BCRM in order to take part in the study.
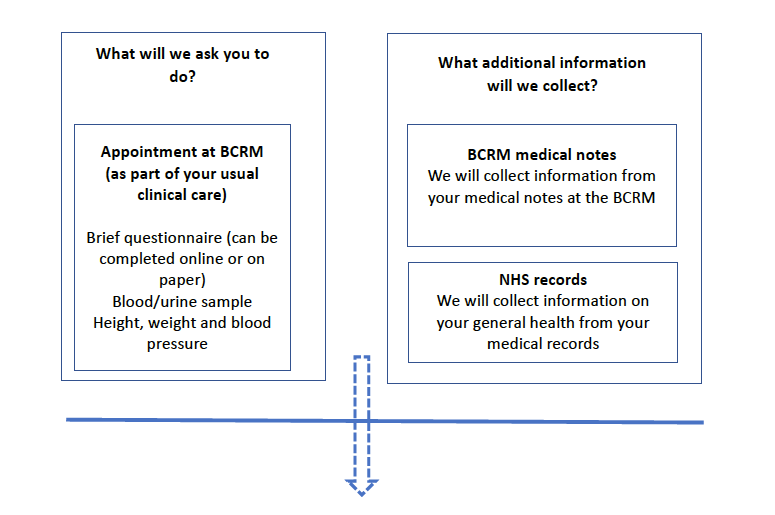
The research nurse will explain to you about the study and answer any questions that you might have. If you agree to participate in this study, we will first ask you to sign the consent form. You will be asked to complete a questionnaire (about your general health and lifestyle) that will take about 10 minutes. We will also measure your height, weight, blood pressure and we will obtain a blood sample. Where possible, we will take this at the same time as your routine blood tests. We will also ask you to provide a urine sample. If you are not able or would prefer not to provide a blood sample, we will ask if you are able to provide a saliva sample, from which we can extract DNA. We will let you know if your blood pressure is outside the range considered normal and give you a letter to take to your doctor as they may wish to check it again. If you decline to consent to be informed, we would not be able to tell you if your blood pressure was found to be outside the range considered to be normal. We will however inform your GP. If your blood pressure was found to be dangerously high or low, needing urgent medical attention, then we must inform you.
The machine we use to measure your blood pressure also measures your pulse rate at the same time. We do not collect pulse rate as part of the study measures but we will let you know if your pulse rate is outside the range considered normal and give you a letter to take to your doctor as they may wish to check it again.
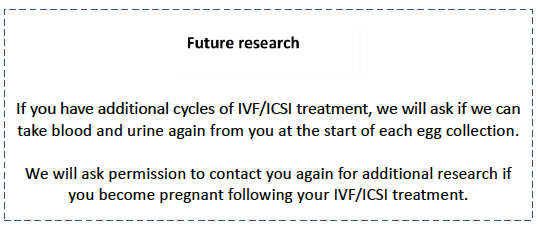
If you undergo more than one egg collection, we would like to take an additional blood and urine sample. We won’t ask for additional samples from partners at this stage. We will seek additional consent for these samples.
We will ask to access your and your partner’s medical notes at the BCRM and in the NHS (via data linkage – see below) to obtain information about your IVF/ICSI treatment including outcomes and your reproductive and general health. Our research midwives may also ask you directly for information about your IVF/ICSI treatment and your reproductive history and general health.
What is data linkage?
If you consent to linkage, we would use your name, address and date of birth to request access to your electronic records or to request copies of any paper records. We can request these with your permission.
We would like to link to your NHS health records. These records include information such as:
- details of visits to your doctor and any medicine you were given
- if you’ve ever been to hospital; why you were there and what happened while you were there
- information about you (e.g. ethnic group)
- your contact details, if we lose touch.
By linking to your health records, we can accurately record the technical information we need. We will collect your records on a regular basis into the future, unless you tell us to stop. Once we have obtained your health records, we will remove your personal details and replace them with a unique identification number. If you would like us to stop collecting this information, please email us at ivf-study@bristol.ac.uk.
We would like to use this information, along with any information about you that we have already collected, for research purposes only. The BRIST-IVF Study is not legally allowed to let you see what is in your official records so we will not feedback any information to you. You do have the right to do this directly with the official organisations that hold your records.
What will you do with my samples?
We plan to use your samples to look at genetic factors (such as DNA) which may be associated with IVF success and longer-term outcomes. We would also like to look at epigenetics, which is a measure of the degree to which our genes are “switched on or off” and to measure levels of metabolites such as fats and sugars in blood and urine. There is some evidence that epigenetic factors may differ in children conceived via IVF compared to those conceived naturally, but relatively little is known about this.
In addition, we will store your samples for use in future research. The questions being asked by health researchers are constantly changing and we are in a position to be able to respond quickly to these questions by having samples ready for analysis.
Your samples will be stored initially at the BCRM and North Bristol NHS Trust before being transferred to the Bristol Bioresources Laboratories at the University of Bristol for processing and storage. Samples will be labelled with a unique ID number but will not be labelled with identifying information such as your name, address and date of birth. The laboratories analysing the samples will not have any access to personal information about you. A lot of the research using your samples will take place in Bristol but some of your samples may be made available to researchers working in universities, hospitals or other organisations in the UK or abroad. We may ask for a fee from researchers to help cover the costs of storing your samples as well as the costs associated with sending them to other places. We will not sell or make any profit from the samples or cell lines you donate and they will only be used in ethically approved research.
Will you feedback any information about my/our samples?
No. The samples we are taking from you will not be used for diagnostic purposes and will not provide information that is clinically relevant to you. It will not be fed back to the clinicians who are treating you. The information from your samples will be used alongside the information from other individuals undergoing IVF/ICSI treatment to help us to identify factors which may contribute to IVF success.
What about dissemination of the results?
The results of the study will be written up in peer-reviewed scientific journals and scientific conference presentations. A summary of the research findings will be made available to the participants on completion of the project or on the publication of any papers.
If I/we are using donor sperm for IVF treatment, why do you want to collect biological samples from both partners?
We are interested in the impact of IVF treatment on health of all individuals undergoing IVF treatment. In addition, we do not necessarily know whether associations between maternal and paternal factors and IVF outcomes are due to biological processes or whether they are a result of shared environmental factors. By comparing biological and other information on parents who are not biologically related to their child as well as those who are, we are better able to identify biological processes that may affect treatment outcomes.
Are there any risks for me in taking part in this study?
No. You will follow your planned treatment protocol and the study does not ask you to adopt any changes to your treatment plan.
What are the benefits?
The potential results from this study will provide information about the impact of maternal and partner health on IVF/ICSI outcome.
What are the costs for taking part in this study?
There will be no cost for taking part in this study.
Who is organising and funding this study?
This study is being funded by the National Institute for Health Research (NIHR) and conducted by the NIHR Biomedical Research Centre at the University of Bristol.
Who has reviewed this study?
The study procedures have been checked, reviewed and agreed by a National Health Service Research Ethics Committee (NHS REC).
Will my/our information be kept confidential?
Yes. The University of Bristol is the sponsor for this study based in the United Kingdom. We will be using information from you and your medical records in order to undertake this study and will act as the data controller for this study. This means that we are responsible for looking after your information and using it properly. The University of Bristol will keep identifiable information about you for 10 years after the study has finished.
There are several ways in which you may choose not to participate in the study. You are able to participate but opt out of any specific types of data collection/linkage at the time of recruitment or at any time as the study progresses. After recruitment, you can ask not to be contacted for any further follow up but be happy for us to use data we have already collected for research purposes. You can decide to withdraw completely so that we do not contact you for further follow up or use the data that we have collected for research purposes. Please contact us at ivf-study@bristol.ac.uk if you would like to withdraw from the study at any time.
Your rights to access, change or move your information are limited, as we need to manage your information in specific ways in order for the research to be reliable and accurate. If you withdraw from the study, we will keep the information about you that we have already obtained, but we will no longer use it for research purposes. To safeguard your rights, we will use the minimum personally identifiable information possible.
The Bristol Centre for Reproductive Medicine will collect information from you and your medical records for this research study in accordance with our instructions. The University of Bristol will use your name and contact details to contact you about the research study. Individuals from the University of Bristol and regulatory organisations may look at your medical and research records to check the accuracy of the research study. The Bristol Centre for Reproductive Medicine will pass these details to the University of Bristol along with the information collected from you and your medical records. The only people in the University of Bristol who will have access to information that identifies you will be people who need to contact you about future data collection or audit the data collection process. The people who analyse the information will not be able to identify you and will not be able to find out your name or contact details.
The University of Bristol will collect information about you for this research study from your medical records. This information will include your name and contact details and health information, which is regarded as a special category of information.
When you agree to take part in a research study, the information about your health and care may be provided to researchers running other research studies in this organisation and in other organisations. These organisations may be universities, NHS organisations or companies involved in health and care research in this country or abroad. Your information will only be used by organisations and researchers to conduct research in accordance with the UK Policy Framework for Health and Social Care Research. This information will NOT identify you and will not be combined with other information in a way that could identify you. The information will only be used for the purpose of health and care research, and cannot be used to contact you or affect your care. It will not be used to make decisions about future services available to you, such as insurance.
You can find out more about how we use your information at the following link http://www.bristol.ac.uk/secre... or by contacting us at ivf-study@bristol.ac.uk.